To certain patients, elimination may mean freedom from extra burden. To others, elimination means freedom from reinfection.
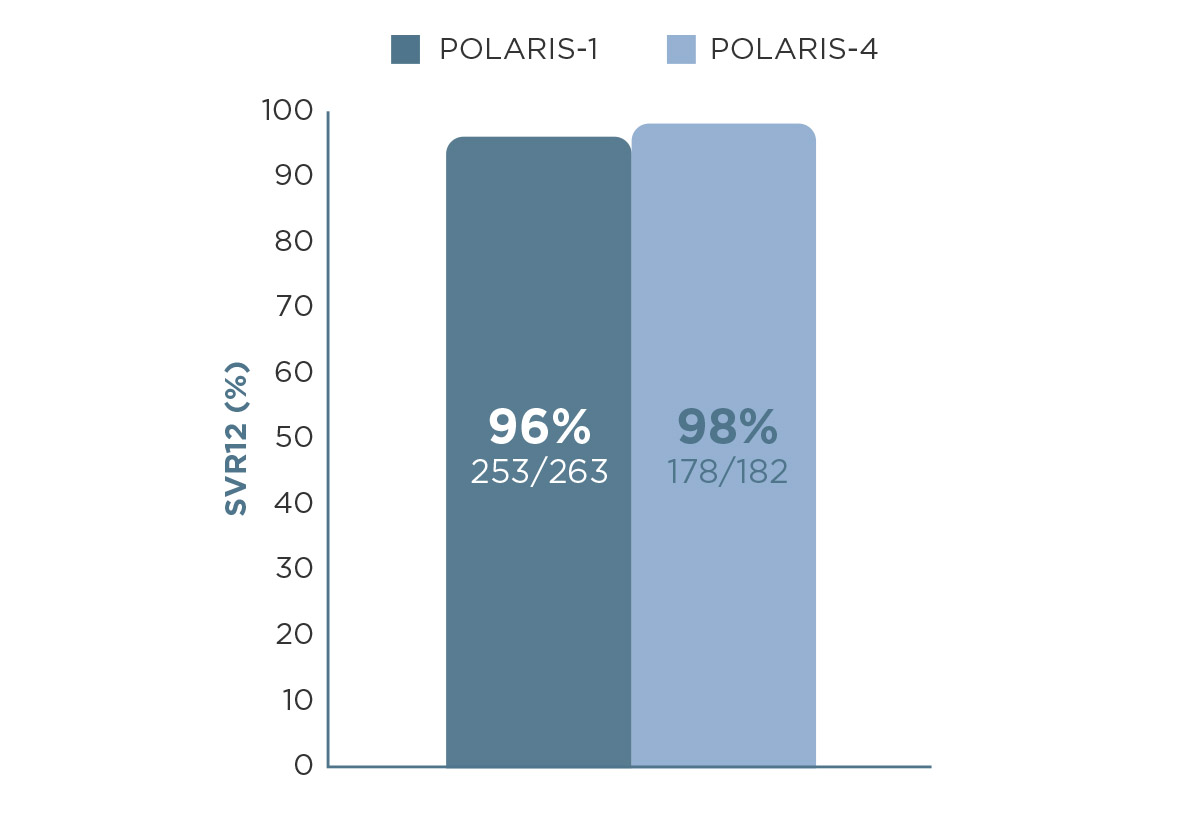
EPCLUSA® (sofosbuvir and velpatasvir) delivers high cure (94%, n=97/103) even though real-world settings can vary.4 And with VOSEVI®, you can offer patients a treatment specifically licensed after DAA failure.1,5
EPCLUSA® and VOSEVI® offer a simplified route to HCV elimination.
EPCLUSA® (sofosbuvir and velpatasvir 400 mg/100 mg tablets)
EPCLUSA® is indicated for the treatment of chronic hepatitis C virus (HCV) infection in patients 3 years of age and older.