DAA = direct-acting antiviral; EASL = European Association for the Study of the Liver; NS5A = nonstructural protein 5A; RAS = resistance-associated substitution; SVR = sustained virologic response.
The EASL 2020 recommendations
Below is a summary of the 2020 EASL recommendations for retreatment after patients experience failure on DAA treatments.1
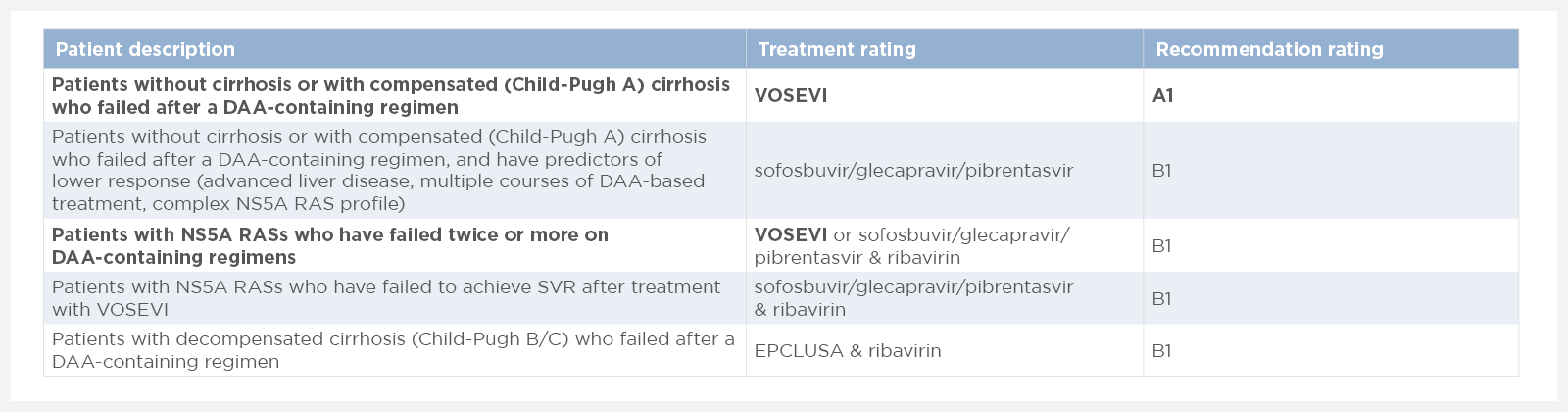
Adapted from EASL.1 Please refer to individual SmPCs prior to prescribing2,3
EPCLUSA® (sofosbuvir and velpatasvir 400 mg/100 mg tablets)
EPCLUSA® is indicated for the treatment of chronic hepatitis C virus (HCV) infection in patients 3 years of age and older.
You may also like to visit
Abbreviations:
References:
- European Association for the Study of the Liver (EASL). J Hepatol. 2020;73:1170–1218.
- VOSEVI® Summary of Product Characteristics.
- EPCLUSA® Summary of Product Characteristics.
UK-VSV-0090
Date of preparation July 2024

