| VOSEVI®’s pan-genotypic efficacy means you can treat with confidence, allowing you to focus on your next patient and support the ambition to leave no patient behind on the path to HCV elimination.1,2 |
Efficacy across HCV genotypes
A treatment that works across HCV genotypes removes unwanted steps for you and your patient on their route to cure.1,2
VOSEVI® demonstrated cure rates (achieving SVR12) of 90% across all HCV genotypes in the POLARIS-1 and POLARIS-4 clinical trials.1,2
EASL defines cure as SVR, i.e. undetectable viral RNA after treatment completion.3
POLARIS-1 was a randomised, double-blind, placebo-controlled, multicentre trial evaluating 12-week treatment with VOSEVI® in patients with HCV genotype 1 (placebo, n=152; VOSEVI®, n=263). POLARIS-4 was a randomised, open-label, multicentre trial evaluating 12-week treatment with VOSEVI® or EPCLUSA® in patients with HCV genotype 1, 2, 3 (VOSEVI®, n=182; EPCLUSA®, n=151). In both trials, patients who were infected with HCV of any other genotype were assigned to receive sofosbuvir–velpatasvir–voxilaprevir for 12 weeks.1,2
|
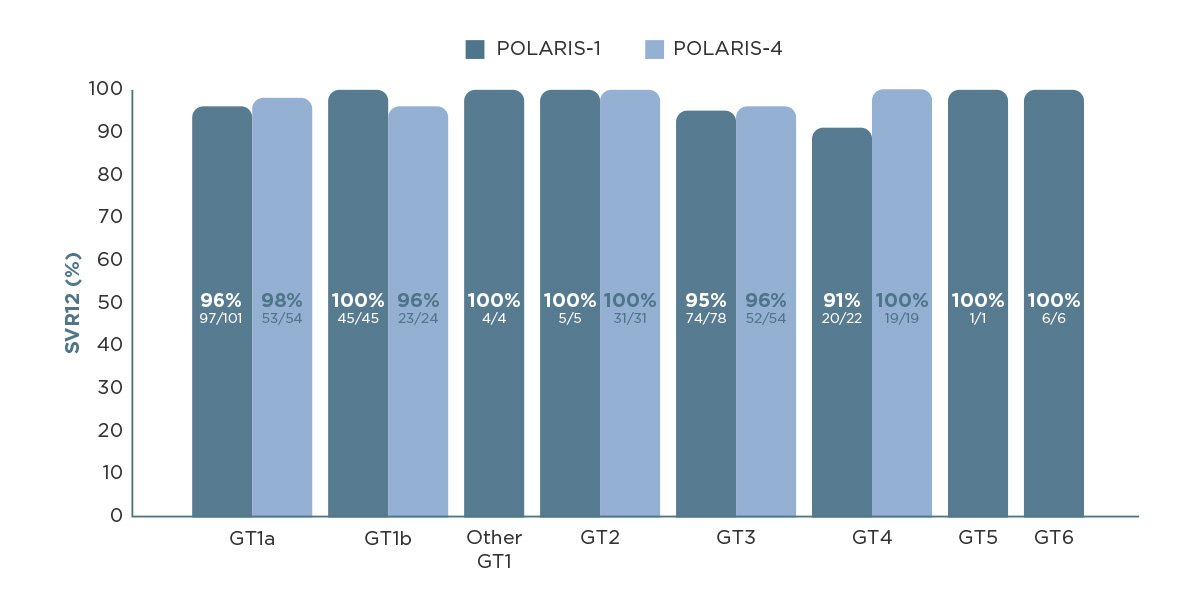
SVR12, sustained virological response at 12 weeks after treatment.
This was backed up by real-world data from a study of over 500 patients experienced with DAA treatment, who received VOSEVI® for 12 weeks. Cure rates (achieving SVR12) of 90–100% were demonstrated across HCV genotypes 1–4, supporting the ambition to leave no patient behind on the path to HCV elimination.4
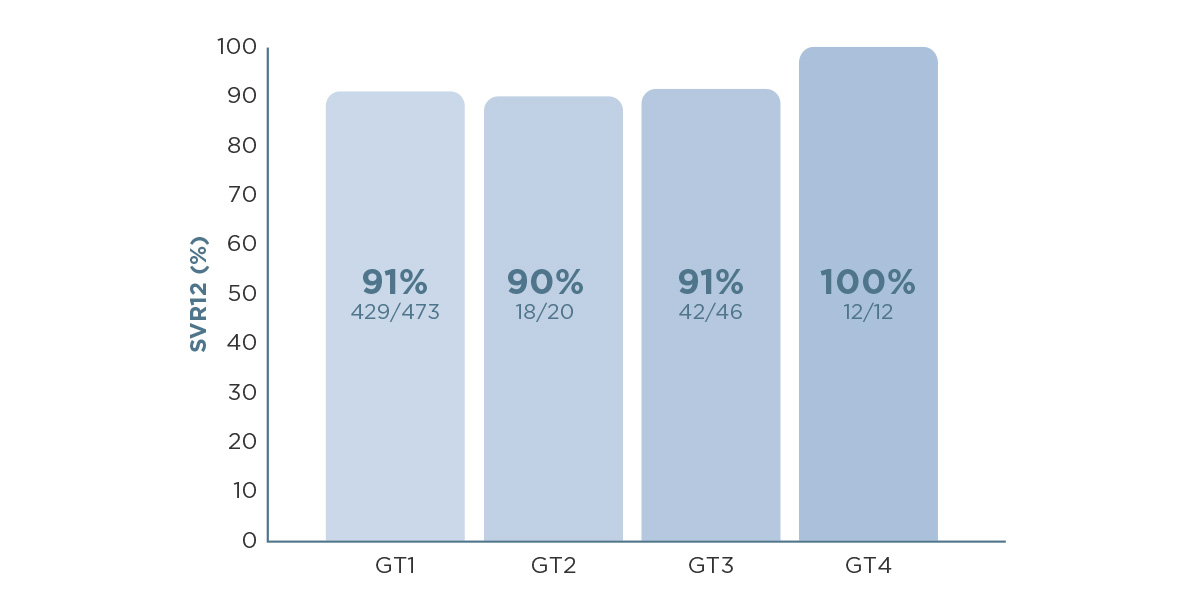
SVR12, sustained virological response at 12 weeks after treatment.
Efficacy in cirrhotic and non-cirrhotic patients
Efficacy in cirrhotic and non-cirrhotic patients
VOSEVI® achieves high cure rates regardless of cirrhotic status in the real world, demonstrating an efficacy (achieving SVR12) of 93.4% (169/181) in cirrhotic patients.4 Cure rates were stable across cirrhotic and non-cirrhotic groups.4
EPCLUSA® (sofosbuvir and velpatasvir 400 mg/100 mg tablets)
EPCLUSA® is indicated for the treatment of chronic hepatitis C virus (HCV) infection in patients 3 years of age and older.
You may also like to visit
Abbreviations:
EASL = European Association for the Study of the Liver; GT = genotype; RNA = ribonucleic acid; SVR = sustained virologic response.
References:
- VOSEVI® Summary of Product Characteristics.
- Bourlière M et al. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med. 2017;376:2134–2146.
- European Association for the Study of the Liver (EASL). J Hepatol. 2020;73:1170–1218.
- Belperio P et al. Real-world effectiveness of sofosbuvir/velpatasvir/voxilaprevir in 573 direct-acting antiviral experienced hepatitis C patients. J Viral Hepat. 2019;26:980–990.
UK-VSV-0090
Date of preparation July 2024

