HCV patients are often feeling tired and worried,2 and may have faced additional challenges, like addiction and comorbidities, on their path to cure.3,*
EASL defines cure as SVR, i.e. undetectable viral RNA after treatment completion.3
Your patients may face unstable lifestyles, making adherence to complex dosing regimens more difficult.3,4
VOSEVI®’s straightforward dosing regimen avoids adding unnecessary complexity to your patients’ lives – keeping your chronic HCV patient on the route to cure, and supporting the ambition to leave no patient behind on the path to HCV elimination. |
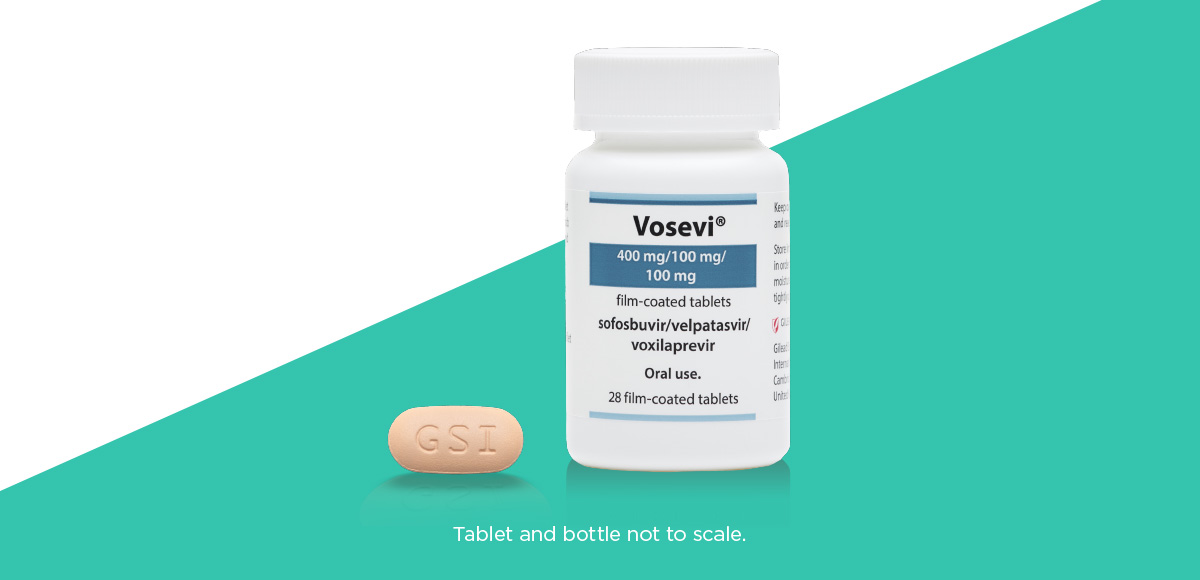
The option of one pill, once a day1
VOSEVI® can be taken as one tablet, once daily, with food.1
You may also like to visit
Footnotes:
| *Concomitant use with medicinal products that are strong P-glycoprotein and/or strong cytochrome P450 inducers, rosuvastin, dabigatran etexilate and ethinylestradiol-containing products are contraindicated with VOSEVI®. VOSEVI® also has multiple interactions with other medicinal products, always refer to the SmPC before prescribing.1 |
Abbreviations:
EASL = European Association for the Study of the Liver; HCV = hepatitis C virus; RNA = ribonucleic acid; SVR = sustained virologic response.
References:
- VOSEVI® Summary of Product Characteristics
- Younossi Z et al. Sofosbuvir and velpatasvir with or without voxilaprevir in direct-acting antiviral-naïve chronic hepatitis C: patient-reported outcomes from POLARIS 2 and 3. Aliment Pharmacol Ther. 2018;47:259–267.
- European Association for the Study of the Liver (EASL). J Hepatol. 2020;73:1170–1218.
- Degenhardt L et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:1385–1398.
UK-VSV-0090
Date of preparation July 2024

