| There were no patients receiving VOSEVI® for 12 weeks who permanently discontinued treatment due to adverse reactions in the Phase 2 and 3 pivotal clinical studies.1 |
Safety profile data
In Phase 2 and 3 clinical trials,* the proportion of patients who permanently discontinued treatment due to adverse reactions was 0.1% for patients receiving VOSEVI® for 8 weeks.1
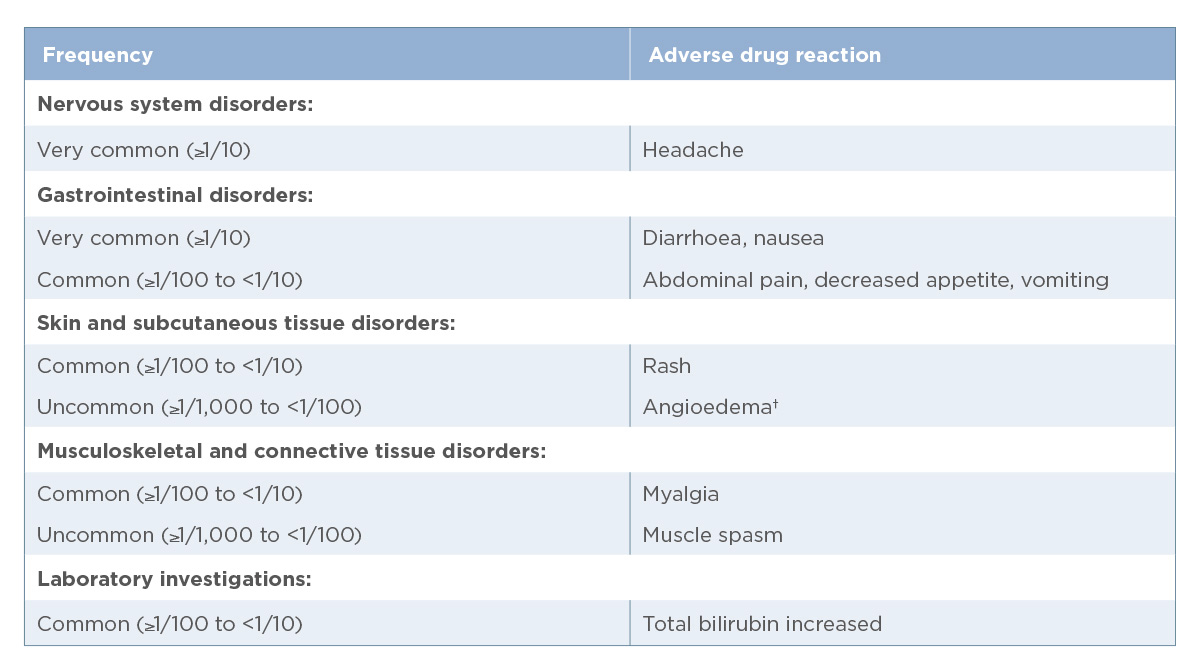
†Adverse reaction identified through safety data from clinical studies and post-marketing experience for sofosbuvir/velpatasvir-containing products.
The observed adverse reactions with the use of VOSEVI® may differ across certain populations, including patients with decompensated cirrhosis and patients with renal impairment.1
Cases of Stevens-Johnson syndrome (frequency not known), severe bradycardia and heart block (when sofosbuvir-containing regimens are used in combination with amiodarone and/or other medicinal products that lower heart rate) have been identified during surveillance of sofosbuvir-containing products.1
Contraindications
Hypersensitivity to active substance or excipients. Concomitant use with strong P-gp and/or strong CYP inducers (e.g. carbamazepine, phenobarbital, phenytoin, rifampicin, rifabutin, and St. John’s wort), rosuvastatin or dabigatran etexilate, and ethinylestradiol-containing medicinal products.1
You may also like to visit
Footnotes:
Abbreviations:
AE = adverse event; CYP = Cytochrome P450; HCV = hepatitis C virus; P-gp = P-glycoprotein.
References:
- VOSEVI® Summary of Product Characteristics.
UK-VSV-0090
Date of preparation July 2024

